Aluminum alloys are widely used in aerospace, automotive manufacturing, rail transit, and architectural decoration due to their low density, high specific strength, corrosion resistance, and excellent processing performance. Melting is the core process in aluminum alloy production, directly determining the internal quality, mechanical properties, and subsequent processing suitability of ingots. In actual production, aluminum alloy melting often faces problems such as low melting efficiency, high energy consumption, severe oxidation loss, gas absorption and inclusion formation, poor compositional uniformity, and coarse grains. These problems not only affect production efficiency and cost control but also reduce the final product yield. This article focuses on the typical problems in aluminum alloy melting, deeply analyzes their formation mechanisms, and proposes targeted countermeasures, providing theoretical and practical references for the optimization of aluminum alloy melting processes.
I. Melting and Efficiency Issues
(1) Problem Manifestations
In the aluminum alloy melting process, low melting efficiency mainly manifests as slow furnace charge melting speed, prolonged melting cycles, and significant variation in melting times between different batches of furnace charge. Taking a common 20-ton gas-fired melting furnace as an example, if melting efficiency is low, the single-furnace melting time may extend from the normal 4–6 hours to 8–10 hours, directly reducing equipment capacity and failing to meet the requirements of continuous production. At the same time, uneven melting is also prominent, with the furnace easily forming “local melting and local clumping” phenomena. Unmelted charge accumulates at the furnace bottom or walls, forming “cold zones,” increasing the difficulty of subsequent holding and homogenization treatment.
(2) Formation Mechanisms
Charge factors: Improper size and shape of furnace charge is a primary factor affecting melting efficiency. Oversized blocks hinder heat transfer through conduction and radiation, leading to surface melting while the core remains solid. If the proportion of fines in the charge is too high, it is easy to form “bridges” in the early melting stage, obstructing furnace airflow and heat transfer. Additionally, fines have a large surface area, significantly increasing oxidation loss. Moreover, surface contaminants such as oil, oxide scales, and moisture absorb a large amount of heat during heating, reducing heat utilization.
Heating method and furnace structure: Different furnace types have significant differences in heating efficiency. For example, reflective furnaces typically have a thermal efficiency of 30%–40%, while induction furnaces can reach 60%–80%. If the heating method is mismatched with the furnace type, such as using side-fired burners in a large furnace, furnace temperature distribution becomes uneven, and the charge is insufficiently heated. Poor insulation performance of the furnace also causes severe heat loss from the walls and roof, resulting in prolonged melting time.
Charging process issues: Improper charging sequence and distribution directly affect melting efficiency. Placing large, heavy blocks directly at the furnace bottom with fines on top reduces the heat transfer area at the bottom, slowing melting. Overloading the furnace reduces voids between charge pieces, preventing effective heat penetration and further lowering melting efficiency.
(3) Countermeasures
Optimize charge pretreatment and ratio
Classify furnace charges according to furnace inlet size and heating power, controlling the block size generally within 50–200 mm, avoiding excessively large or small pieces. Clean the surface of the charge through shot blasting or sandblasting to remove oxide scale and oil. Use drying equipment to control moisture content below 0.05%, reducing heat loss during heating. Reasonably mix return scrap and new material, generally controlling return scrap at 30%–50%, crushing it to appropriate size and uniformly mixing with new material.
Improve heating method and furnace structure
Prefer induction melting furnaces or regenerative combustion furnaces. Induction furnaces use electromagnetic induction to heat the charge internally, achieving fast and uniform temperature rise. Regenerative combustion technology recovers flue gas heat, improving fuel utilization and reducing energy consumption. Optimize the furnace insulation layer using refractory fiber, lightweight refractory bricks, and other high-efficiency insulation materials to reduce heat loss. Install sealing devices on furnace doors and roof to prevent cold air from entering and maintain stable furnace temperature. Employ multiple burner layouts, adjusting nozzle angles and power to achieve uniform temperature distribution and eliminate “cold zones.”
Standardize charging and melting operations
Establish a scientific charging sequence: first lay a 100–150 mm thick layer of fines at the furnace bottom as a “starter layer,” then evenly distribute large and heavy blocks on top, and finally scatter fines to fill voids, avoiding the formation of “bridges.” Strictly control furnace loading, generally maintaining a loading coefficient of 0.7–0.8, ensuring sufficient voids for furnace airflow. During melting, use mechanical or electromagnetic stirring to break oxide films on the charge surface, accelerate heat transfer, and promote uniform melting of the charge.
(1) Problem Manifestations
Aluminum alloy melting is a high-energy-consuming process. The energy consumption per ton of aluminum ingot is usually 50–80 m³/tAl; if the process is not properly controlled, energy consumption may exceed 100 m³/tAl. High energy consumption not only increases production costs but also leads to energy waste and environmental pollution. Excessive energy consumption is often accompanied by large furnace temperature fluctuations and prolonged melting cycles, affecting the stability of ingot quality.
(2) Formation Mechanisms
Severe heat loss: Heat loss is the main cause of high energy consumption, including heat lost through furnace walls, flue gas, and the heating process of the charge. Flue gas alone accounts for 30%–50% of total energy consumption. Directly venting flue gas wastes a large amount of residual heat.
Unreasonable melting process parameters: Excessively high melting temperature or prolonged holding time significantly increases energy consumption. Aluminum alloy melting point is generally around 660℃. Overheating usually controlled at 720–760℃; temperatures above 780℃ not only increase oxidation loss and gas absorption risk but also dramatically increase energy consumption. Long holding time results in continuous furnace heat loss.
Aging equipment and poor maintenance: Long-term use of the furnace erodes and thins furnace lining, reducing insulation performance. Burners or induction coils age, lowering thermal efficiency. Damaged seals on furnace doors or flue allow cold air intrusion, increasing energy consumption.
(3) Countermeasures
Waste heat recovery
Use regenerative combustion systems to recover flue gas heat and preheat combustion air, raising air temperature to 200–500℃, significantly reducing fuel consumption, with energy savings of 20%–30%.
Install waste heat boilers in flue ducts to generate steam for drying furnace charges, heating workshops, or electricity generation, achieving cascaded energy utilization.
Optimize melting process parameters
Strictly control melting temperature according to alloy grade; avoid overheating. For example, 6063 aluminum alloy: 720–740℃; 2024 aluminum alloy: 740–760℃.
Control holding time to 30–60 minutes post-melting to ensure compositional homogenization and purification.
Adopt “fast heating, short holding” to shorten melting cycles and reduce heat loss.
Strengthen equipment maintenance
Regularly inspect and repair furnace lining; rebuild lining when thickness decreases to 1/3 of original.
Clean burners and induction coils; check and maintain seals; replace aged parts.
Implement energy consumption monitoring system to track per-furnace and per-ton energy usage, analyze abnormalities, and adjust process parameters.
(1) Problem Manifestations
Aluminum alloys are highly reactive metals and readily form Al₂O₃ oxide during melting. When the oxide film breaks, the exposed aluminum continues to oxidize, forming oxide inclusions. Oxidation loss is typically 2%–5%, but may exceed 8% if the process is uncontrolled. Oxidation loss reduces metal yield and increases production cost while raising oxide inclusion content, affecting ingot purity and mechanical performance.
(2) Formation Mechanisms
Thermodynamic and kinetic factors: Aluminum has strong affinity for oxygen. Standard Gibbs free energy is low; the reaction proceeds spontaneously at melting temperatures: 4Al + 3O₂ → 2Al₂O₃. High temperature, increased melt surface area, and high oxygen content accelerate the reaction.
Furnace gas composition: Oxygen, water vapor, CO₂ in furnace atmosphere are oxidizing gases. Water vapor reacts with aluminum: 2Al + 3H₂O → Al₂O₃ + 3H₂, causing oxidation loss and hydrogen absorption.
Melting operations: Excessive stirring or violent melt agitation increases aluminum-gas contact area, accelerating oxidation loss. Overheating increases oxidation rate and reduces oxide film stability, forming more inclusions.
(3) Countermeasures
Protective melting atmosphere
Inert gases (N₂, Ar) to isolate melt from air; flow rate 0.5–1.0 m³/h·t.
Reducing gases (CO, natural gas) to lower oxygen content; control concentration to avoid carbon inclusion.
Covering flux (KCl, NaCl, cryolite) to form protective film; dosage 0.3%–0.5% of melt mass.
Optimize operations
Control temperature to avoid overheating; moderate stirring to reduce melt-gas contact.
Use “low-temperature charging, fast melting” to shorten high-temperature exposure.
Skim oxide promptly to prevent entrainment.
Select appropriate fluxes and refining agents
Choose fluxes with covering and refining functions; absorb oxide inclusions.
Avoid fluxes with high oxygen content.
(1) Problem Manifestations
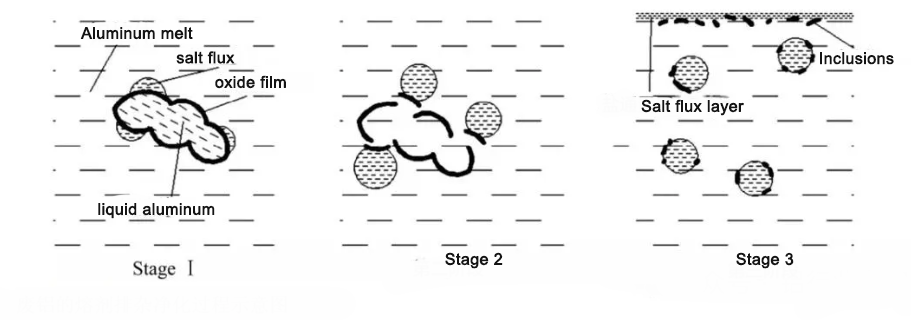
Aluminum melts readily absorb hydrogen, the most common gaseous impurity. Hydrogen solubility increases with temperature. Upon solidification, solubility drops rapidly; excess hydrogen forms pores and pinholes, reducing ingot density and mechanical properties. Non-metallic inclusions (Al₂O₃, MgO, SiO₂) disrupt melt continuity, reducing processing and service performance.
(2) Formation Mechanisms
Hydrogen absorption: Sources include furnace water vapor, moisture in charge, crystallization water in flux. Hydrogen enters melt via adsorption–dissolution: water reacts → hydrogen adsorbs → diffuses into melt. Solubility rises with temperature: 0.23 mL/100g Al at 700℃; 0.41 mL/100g Al at 800℃.
Inclusion formation: Endogenous (from oxidation of melt and alloy elements); exogenous (charge impurities, lining spall, residual flux). Fine inclusions difficult to remove by gravity.
(3) Countermeasures
Degassing
Inert gas bubbling (N₂, Ar); microbubbles via rotary injection; efficiency 30–80%.
Vacuum refining; efficiency >80%, suitable for high-end alloys.
Flux refining (ZnCl₂, MnCl₂) produces volatile gases, aiding hydrogen removal.
Inclusion removal
Gravity settling: 30–60 min hold, skim large inclusions.
Filtration: ceramic foam, 10–30 ppi; fiberglass filters.
Electromagnetic purification: Lorentz force aggregates inclusions for removal.
Source control
Dry charge (120–150℃, ≥2h); bake flux.
Use corrosion-resistant lining; clean furnace bottom slag regularly.
(1) Problem Manifestations
Alloying adjusts elements like Si, Mg, Cu, Mn. Poor uniformity causes local content deviations; e.g., 6063 alloy Si content 0.2–0.6%, may fall outside. Alloy element loss and low alloying efficiency affect stability.
(2) Formation Mechanisms
Element loss and segregation: Mg highly reactive; Si, Cu less so. Slow diffusion and poor stirring cause local concentration.
Improper addition: Direct addition to high-temperature melt increases oxidation; large alloy blocks dissolve slowly.
Insufficient temperature and holding: Low temperature slows diffusion; short holding prevents uniform distribution.
(3) Countermeasures
Optimize addition method and sequence
Use intermediate alloys (Al-Mg, Al-Si, Al-Cu) to reduce oxidation and improve alloying.
Sequence: high-melting, low-loss elements first (Si, Cu); then low-melting, high-loss (Mg, Zn); finally trace elements (Ti, B).
Add in batches with stirring for uniform dissolution.
Stirring and homogenization
Mechanical or electromagnetic stirring 15–30 min.
Hold 30–60 min at 50–80℃ above alloy melting point.
Online homogenization or high-temperature homogenization before rolling for high-quality alloys.
Composition monitoring
Real-time detection with optical spectrometry; adjust additions as needed.
Sample each furnace; establish traceable composition records.
(1) Problem Manifestations
Coarse grains reduce plasticity, toughness, and cause cracking or delamination during subsequent processing. Mechanical properties become uneven, affecting final product quality.
(2) Formation Mechanisms
Excessive superheat: Reduces undercooling, lowers nucleation rate, accelerates grain growth.
Slow cooling rate: Prolongs diffusion time, promoting coarse grains. Thick ingots or poor mold cooling exacerbate coarse grains.
Lack of grain refiners: Absence or insufficient dosage prevents formation of heterogeneous nucleation cores.
(3) Countermeasures
Add grain refiners
Ti-B refiners (Al-Ti-B, Ti-B wires), 0.02–0.05% of melt. TiAl₃ and TiB₂ act as nucleation cores.
Ti-C refiners for Mg-rich alloys.
Preheat to 150–200℃; stir to disperse evenly.
Control superheat and cooling
Superheat 60–100℃ above melting point.
Optimize cooling: water-cooled molds, increase flow, stepwise cooling to ensure uniform internal/external cooling.
Modification treatment
Al-Si alloys: Na or Sr modifiers; 0.01–0.03% Sr transforms coarse plate-like Si into fine spheroids, indirectly refining Al matrix.
VII. Inclusion Issues
(1) Problem Manifestations
Inclusions (endogenous: Al₂O₃, MgO; exogenous: dirt, lining spall, residual flux) reduce tensile strength, elongation, and create stress concentration points, causing cracks during processing.
(2) Formation Mechanisms
Endogenous: Oxidation during melting.
Exogenous: Furnace charge impurities, lining erosion, flux residues.
Improper operation: Excessive stirring, incomplete skimming, spalling during casting.
(3) Countermeasures
Source control: Clean and dry charge; use corrosion-resistant lining; optimize flux.
In-process removal: Combined refining: inert gas bubbling → filtration (ceramic foam 10–30 ppi) → skimming 20–30 min; online refining for continuous purification.
Casting optimization: Bottom/side pouring; preheat gating; clean equipment.
Aluminum alloy melting involves heat transfer, mass transfer, chemical reactions, and phase changes. Efficiency, energy consumption, oxidation loss, gas absorption, composition uniformity, grain structure, and inclusions are interrelated. High temperatures increase energy consumption, oxidation, and gas absorption; strong stirring improves uniformity but increases oxide inclusions.
Resolving typical problems requires a comprehensive approach: raw material pretreatment, equipment optimization, process control, and standardized operations. With high-performance, high-purity alloys and green manufacturing, melting technology trends toward high efficiency, low energy, pollution-free, and intelligent control. Advanced systems allow precise control of temperature, pressure, and gas flow; new refining technologies (laser, ultrasonic) improve melt purity; innovative waste heat recovery reduces energy consumption. Continuous technological innovation and process optimization will significantly enhance the quality and efficiency of aluminum alloy melting, supporting high-quality development of the aluminum industry.
Post time: Jan-01-2026