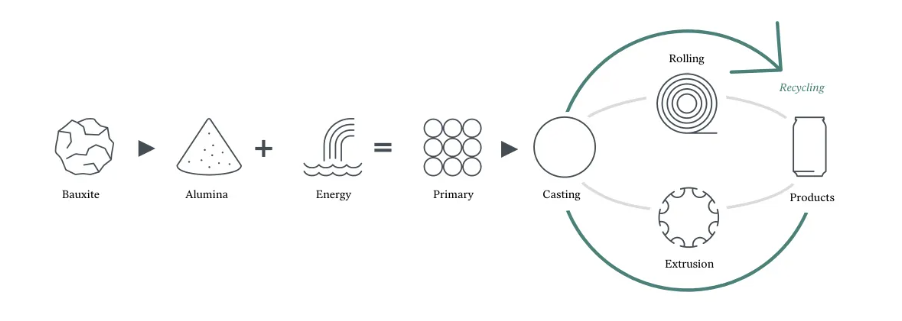
In the vast system of modern industry, aluminum, as an important metal material, is widely used in construction, automobiles, packaging, electronics and many other fields due to its light weight, high strength and excellent corrosion resistance. It profoundly influences people’s daily lives and global economic development. With such extensive applications, how does aluminum transform from deeply buried bauxite into common products such as beverage cans and aluminum sheets? Behind this lies a complex and delicate full industrial chain, covering bauxite mining, alumina production, electrolytic aluminum smelting, aluminum material processing and aluminum product manufacturing, and recycling of waste aluminum. Next, let us explore the origin and developmental trajectory of aluminum.
I. Bauxite Mining: The Beginning of Aluminum’s Journey
Bauxite is the main raw material for aluminum production. Global bauxite resources are abundant and widely distributed, mainly in Guinea, Australia, Brazil and Jamaica. China also has certain reserves, mainly in Guangxi, Guizhou and Henan. Bauxite mining is mainly divided into open-pit mining and underground mining, with open-pit mining dominating globally due to its lower cost and higher efficiency.
Before mining, detailed geological exploration is required, using techniques such as geological mapping and drilling to precisely determine reserves, grade and distribution, as well as analyze ore characteristics. Based on these exploration data, mining areas, transportation routes and equipment layout can be rationally planned.
After preparation, actual mining begins. Bulldozers and excavators are used to remove vegetation and topsoil, and temporary roads and necessary facilities are built. Large mining equipment such as electric shovels and hydraulic shovels then extract the ore, which is transported by trucks or belt conveyors to designated sites for preliminary processing.
Mined bauxite often contains impurities such as iron, silicon and titanium, requiring beneficiation to improve the grade. Common beneficiation methods include crushing, screening, grinding and flotation to remove impurities and obtain concentrate that meets alumina production requirements.
II. Alumina Production: The Transformation from Ore to Basic Raw Material
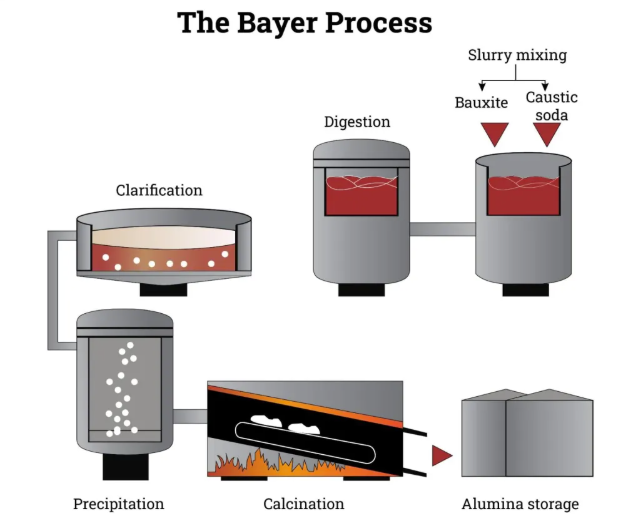
Bauxite that has undergone mining and beneficiation is transported to alumina plants for alumina production. Currently, the main industrial method is the Bayer process, in addition to the sintering process and the Bayer–sintering combined process.
(1) Bayer Process
The Bayer process is the most widely used method for alumina production. Its principle is that sodium hydroxide solution dissolves alumina in bauxite under high temperature and pressure, forming sodium aluminate solution.
Reaction: Al2O3 + 2NaOH + 3H2O = 2NaAl(OH)4
The sodium aluminate solution is diluted and settled to remove insoluble residues (red mud). Then aluminum hydroxide seed crystals are added, and the solution is cooled and stirred to precipitate aluminum hydroxide.
Reaction: NaAl(OH)4 = Al(OH)3↓ + NaOH
The aluminum hydroxide precipitate is filtered, washed and calcined at high temperature to obtain alumina.
Reaction: 2Al(OH)3 = Al2O3 + 3H2O
The Bayer process has advantages such as simple flow, low cost and good product quality, and is suitable for high-grade bauxite (Al/Si ratio > 7). However, its ability to process low-grade bauxite is limited.
(2) Sintering Process
The sintering process is suitable for bauxite with low Al/Si ratio (less than 5). Bauxite, limestone and soda ash are mixed in a certain ratio and sintered at high temperature, where alumina reacts with soda ash to form sodium aluminate.
Reaction: Al2O3 + Na2CO3 = 2NaAlO2 + CO2↑
The clinker is ground and dissolved, allowing sodium aluminate to enter solution, and then undergoes desilication, carbonation and other steps to obtain aluminum hydroxide, which is calcined to produce alumina.
The sintering process has strong adaptability to low-grade bauxite but has high energy consumption and complex procedures.
(3) Bayer–Sintering Combined Process
This method combines the advantages of both processes and includes parallel, series and mixed routes.
The parallel route treats the Bayer process and sintering process as two independent systems, with sodium hydroxide solution from sintering supplementing the Bayer system.
The series route uses the Bayer process first for high-grade bauxite, and the resulting red mud is further treated by sintering to recover alumina.
The mixed route adds some low-grade bauxite into the sintering system based on the series route to increase capacity.
This combined method improves resource utilization and reduces production cost.
The alumina produced is a white powder with high hardness, high melting point and stable chemical properties, and is the key raw material for aluminum electrolysis.
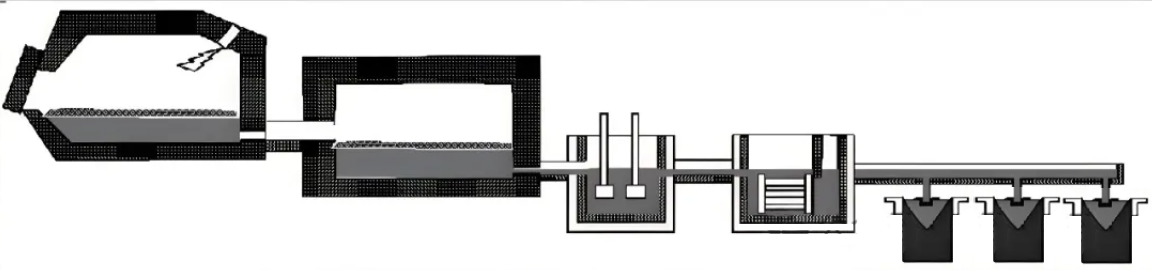
III. Electrolytic Aluminum Production: Achieving the Metallic Transformation of Aluminum
Although alumina is an important aluminum compound, it must be further processed to obtain metallic aluminum. The only industrial method is the cryolite–alumina molten salt electrolysis process, also called the Hall–Héroult process.
Alumina has an extremely high melting point (about 2050°C), making direct melting impractical. Cryolite (Na3AlF6), with a much lower melting point (about 1008°C), can dissolve alumina and form a conductive molten electrolyte, enabling electrolysis at a lower temperature.
Electrolysis is carried out in electrolytic cells using carbon anodes and cathodes. Alumina is dissolved in molten cryolite. When direct current is applied:
At the anode, oxygen reacts with carbon to form CO2 and CO.
C + O2 = CO2↑
2C + O2 = 2CO↑
At the cathode, aluminum ions gain electrons and are reduced to liquid aluminum, which collects at the bottom of the cell.
To ensure stable operation, temperature, electrolyte composition, cell voltage and current must be strictly controlled. Alumina and carbon anodes must be replenished continuously.
Electrolysis produces large amounts of fluorides and dust. China’s aluminum plants widely use dry scrubbing, where alumina adsorbs hydrogen fluoride. Purified gas is emitted, with fluoride removal rates above 98%.
The resulting aluminum typically has purity above 99% and can be processed into ingots, billets, rolling slabs, rods and other forms for further aluminum processing.
IV. From Electrolytic Aluminum to High-Precision Aluminum Sheet and Strip: The Advanced Path of Aluminum Processing
Aluminum billets require further processing to become high-precision aluminum sheets and strips. The processing involves melting, casting, rolling and heat treatment.
(1) Melting and Casting
Electrolytic aluminum billets or recycled aluminum are melted. Alloying elements such as copper, magnesium, silicon and manganese are added as required.
The molten aluminum is refined to remove gases and impurities through gas stirring, powder refining or filtration. When temperature and composition meet specifications, the melt is cast into slabs or billets. Casting temperature, speed and cooling must be strictly controlled.
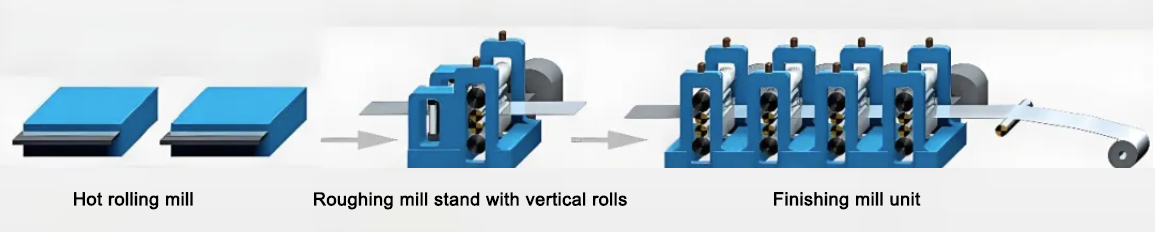
(2) Rolling
After surface milling and homogenization, slabs enter the rolling stage. Rolling reduces thickness and increases width and length.
Hot rolling at 400–500°C improves internal structure, eliminates defects and enables large deformation.
Cold rolling at room temperature further improves surface quality, precision, grain refinement and strength. Lubricants reduce friction. Cold rolling can produce very thin sheets, such as 0.006–0.2 mm.
(3) Heat Treatment
Heat treatment further improves performance such as strength, hardness, toughness and corrosion resistance. Common processes include:
Annealing: heating and slow cooling to remove work hardening and restore plasticity.
Solution treatment: heating to dissolve alloying elements and quenching to form supersaturated solid solution.
Aging: heating to allow precipitation strengthening; includes natural aging and artificial aging.
Through these processes, aluminum sheet and strip achieve high performance for aerospace, automotive, building decoration and electronics. Aerospace aluminum alloys must have high strength, low density, corrosion resistance and fatigue resistance, achieved by precise process control.
V. Aluminum Product Manufacturing: Aluminum Enters Everyday Life
High-precision aluminum sheet and strip become raw materials for various aluminum products. Aluminum cans are a typical example.
Can manufacturing includes stamping, drawing, ironing, necking and bottom sealing. Aluminum sheets are cut into round blanks, which are drawn into cups. The cups undergo multiple ironing steps to reduce wall thickness and increase height, with lubricants used throughout.
The can body is necked to reduce the opening diameter, and the bottom is sealed using resistance welding or laser welding. Finished cans are sent to beverage companies for filling, labeling and packaging. Besides cans, aluminum is widely used in automotive parts, doors and windows, electronics housings, furniture and more.
VI. Recycling Waste Aluminum Cans: Aluminum’s Circular Rebirth
As consumption increases, waste aluminum cans also grow in quantity. Recycling reduces waste, lowers pollution and enables circular use of aluminum.
Recycling includes collection, pretreatment and remelting. Waste cans are collected and sorted to remove impurities such as plastic, paper and iron.
Pretreatment includes cleaning, crushing and de-coating to improve recycling efficiency. The treated fragments are melted and refined to remove impurities. The molten aluminum is cast into ingots or billets for reuse in new products.
High-grade recycling aims to preserve aluminum quality so it can again be used for high-value products such as can stock. Some advanced companies now achieve full-process high-grade recycling, turning waste cans into high-quality can-sheet billets.
From bauxite mining to recycling aluminum cans, the full aluminum industry chain demonstrates rational resource utilization. It drives global industry development, meets demand and contributes to resource conservation and environmental protection. With continuous technological progress, each stage of the aluminum industry will continue to innovate, and aluminum will show greater value for sustainable development.
Post time: Nov-16-2025