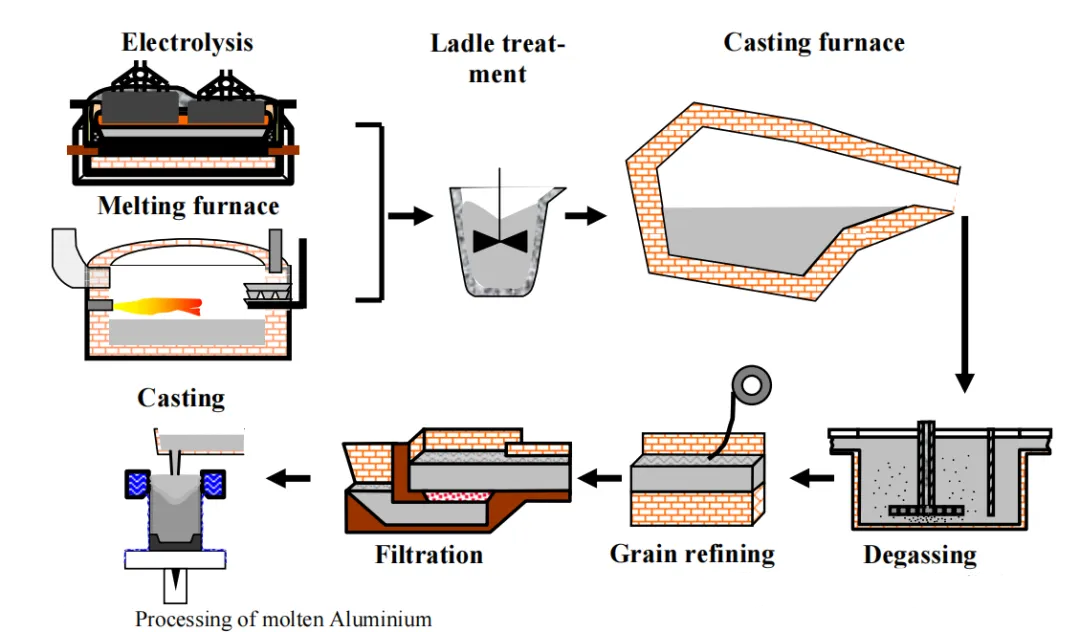
Aluminum alloy melt treatment (Melt Treatment of Aluminium) is a core process in the aluminum processing industry chain, directly determining the internal quality, mechanical properties, and service life of castings and wrought products. As the critical link connecting raw material melting and final forming, melt treatment eliminates harmful defects such as hydrogen and non-metallic inclusions through a series of processes including degassing, inclusion removal, composition homogenization, and grain refinement. These processes optimize the metallurgical state of the melt and provide high-quality molten metal for subsequent processing.
This paper systematically elaborates on the technical principles, key process routes, core equipment, and industrial application status of aluminum alloy melt treatment. Special emphasis is placed on the technical challenges and innovation directions of recycled aluminum melt treatment, aiming to provide references for technological upgrading and high-quality development of the aluminum industry.
1. Introduction
Aluminum alloys, owing to their low density, high specific strength, excellent corrosion resistance, and good processability, have been widely applied in high-end manufacturing fields such as automotive, aerospace, consumer electronics (3C), and new energy industries. With the advancement of the “dual-carbon” policy and increasing demands for industrial upgrading, the market has imposed increasingly stringent requirements on the quality of aluminum alloy products. In addition to higher mechanical performance, extremely strict control over internal defects such as porosity and inclusions is required.
The quality of molten aluminum directly determines the performance of the final product. Untreated aluminum melts typically contain 0.15–0.5 ml/100 g Al of hydrogen, as well as non-metallic inclusions such as oxides, nitrides, and carbides. These defects can lead to porosity, cracks, and inclusion-related defects in cast products, severely reducing product yield and service life.
Aluminum alloy melt treatment (Melt Treatment of Aluminium) refers to the entire process carried out after aluminum and aluminum alloys are melted and before pouring or forming, using a series of physical, chemical, or physicochemical methods to remove harmful gases and inclusions, adjust alloy composition, and optimize melt fluidity. The core objectives are to reduce the hydrogen content in the melt to below 0.1 ml/100 g Al, control the size of non-metallic inclusions within 20 μm, and achieve uniform alloy composition distribution and stable melt properties.
As the key quality-enhancing process in aluminum processing, the level of melt treatment technology directly reflects the core competitiveness of enterprises. This is particularly critical in the recycled aluminum industry, where complex raw material compositions and high impurity contents make melt treatment the core technological support for overcoming bottlenecks in high-end recycled aluminum applications.
Starting from fundamental principles, this paper systematically dissects the key process steps of melt treatment, introduces mainstream equipment types and application scenarios, analyzes current technical challenges faced by the industry, and discusses future development trends, providing a comprehensive reference for the research and application of aluminum alloy melt treatment technologies.
2. Technical Principles and Core Objectives of Aluminum Alloy Melt Treatment
2.1 Technical Principles
Harmful defects in aluminum melts mainly originate from the melting process. Hydrogen is primarily generated through reactions between molten aluminum and water vapor or combustion products in furnace atmospheres. The solubility of hydrogen in aluminum decreases significantly with decreasing temperature, and during solidification, hydrogen readily precipitates to form gas porosity. Non-metallic inclusions mainly originate from oxide films introduced by raw materials, erosion products from furnace linings, and residues of refining agents. These inclusions disrupt the continuity of the alloy, act as stress concentration sites, and reduce the strength, ductility, and corrosion resistance of the material.
The technical principles of aluminum alloy melt treatment are based on two core concepts: separation and optimization. Physical or chemical methods are employed to separate gases and inclusions from the molten aluminum, while composition adjustment and microstructural control are used to optimize the physicochemical properties of the melt. Specifically, degassing processes utilize density differences between gases and the melt, changes in gas solubility, or chemical reactions to remove hydrogen from the melt; inclusion removal processes rely on adsorption and flotation mechanisms to aggregate fine inclusions into larger particles that can be separated more easily; composition homogenization and grain refinement are achieved by adding alloying elements and grain refiners to regulate melt composition distribution and solidification structure, thereby enhancing material performance.
2.2 Core Objectives
The ultimate objective of aluminum alloy melt treatment is to provide clean, homogeneous, and stable molten metal for subsequent forming processes. Specifically, this objective can be summarized in the following aspects:
Gas content control: Reducing the hydrogen content in the melt to 0.05–0.1 ml/100 g Al (adjusted according to product grade requirements), in order to prevent hydrogen precipitation during solidification and the formation of gas porosity defects.
Inclusion purification: Removing non-metallic inclusions with sizes larger than 10 μm and reducing the total inclusion content to within specified limits. For example, high-end aerospace castings require an inclusion area fraction of no more than 0.05%.
Performance optimization: Improving melt fluidity through composition homogenization to reduce filling resistance during pouring, and enhancing solidification structure through grain refinement to improve the mechanical properties and processing performance of final products.
3. Key Process Steps of Aluminum Alloy Melt Treatment
Aluminum alloy melt treatment is a complex, multi-process collaborative operation. The core processes include degassing, inclusion removal, composition adjustment, grain refinement, and melt holding. These processes are closely interconnected and must be precisely controlled according to alloy type, raw material characteristics, and product requirements.
3.1 Degassing: Eliminating the “Invisible Killer” Hydrogen
Degassing is one of the most critical processes in melt treatment. Its purpose is to remove dissolved hydrogen from molten aluminum and prevent gas porosity defects in cast products. Hydrogen is regarded as the “invisible killer” affecting aluminum alloy quality. The solubility of hydrogen in molten aluminum is approximately 0.4 ml/100 g Al at 760 °C, while it is only about 0.001 ml/100 g Al at room temperature. During solidification, the sharp decrease in hydrogen solubility leads to massive hydrogen precipitation, forming dispersed gas pores that severely deteriorate material density and mechanical properties.
3.1.1 Degassing Principles
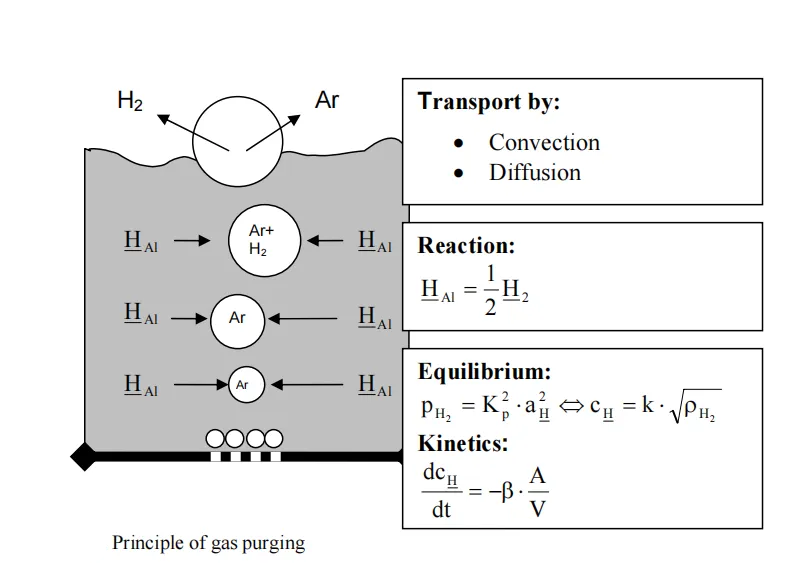
The core principles of degassing are gas replacement and bubble flotation. Inert gases that do not react with molten aluminum, such as argon (Ar) and nitrogen (N₂), or reactive gases such as chlorine (Cl₂), are introduced into the melt to form fine gas bubbles. As these bubbles rise through the molten aluminum, they adsorb dissolved hydrogen. When the bubbles reach the melt surface and burst, hydrogen is released into the atmosphere, thereby achieving degassing.
In addition, certain reactive gases (such as Cl₂) can chemically react with hydrogen dissolved in the melt to form hydrogen chloride (HCl) gas, further enhancing the degassing effect.
3.1.2 Mainstream Degassing Processes
Rotary Degassing
Rotary degassing is currently the most widely used degassing technology in industrial production. In this process, a motor-driven rotating shaft drives a graphite rotor at high speed (typically 300–600 r/min). Argon or nitrogen is injected through gas channels in the rotor into the molten aluminum, where it is dispersed into uniformly distributed fine bubbles with diameters of approximately 1–3 mm.
The high-speed rotation of the rotor not only breaks the gas into fine bubbles but also stirs the melt, increasing the contact area and contact time between gas bubbles and molten aluminum. Degassing efficiency can reach 60%–80%, reducing hydrogen content to 0.05–0.1 ml/100 g Al. This process features stable degassing performance, simple operation, and minimal melt contamination, making it suitable for large-scale production of various aluminum alloys.
Static Degassing
In static degassing, inert gas is uniformly introduced into the bottom of the melt through porous plugs or permeable bricks. Gas bubbles slowly rise through the melt to achieve hydrogen removal. This method has simple equipment and low cost, but relatively low degassing efficiency (approximately 40%–50%). It is mainly used for ordinary castings with less stringent hydrogen content requirements.
Vacuum Degassing
In vacuum degassing, molten aluminum is placed under a vacuum environment. By reducing the partial pressure of hydrogen above the melt surface, dissolved hydrogen rapidly precipitates from the melt. This process has very high degassing efficiency (over 90%) and can reduce hydrogen content to below 0.03 ml/100 g Al. It is suitable for high-end aluminum alloy products such as those used in aerospace applications. However, due to high equipment investment, high energy consumption, and complex operation, its application in general industrial production is limited.
3.1.3 Key Control Parameters
The effectiveness of degassing depends on the coordinated control of multiple parameters.
Gas type: Argon has stable chemical properties and does not react with molten aluminum, making it the most commonly used degassing gas. Nitrogen has lower cost, but at high temperatures it may react with molten aluminum to form AlN inclusions, so strict temperature control is required. Chlorine provides excellent degassing performance but is toxic and must be used together with appropriate environmental protection systems.
Gas flow rate: If the flow rate is too low, insufficient bubbles are generated and degassing efficiency is reduced; if the flow rate is too high, melt splashing and oxidation risks increase. Typically, the flow rate is controlled within 0.5–2 m³/h, depending on furnace capacity.
Rotor speed: Higher rotor speed improves gas dispersion and bubble uniformity, but excessively high speed may entrain air into the melt. Rotor speed is usually controlled within 300–600 r/min.
Treatment time: Insufficient treatment time results in incomplete degassing, while excessive time increases temperature loss and oxidation risk. Typical degassing time ranges from 15 to 30 minutes.
3.2 Inclusion Removal: Purifying the Melt Environment
Inclusion removal is the process of eliminating non-metallic inclusions from molten aluminum. These inclusions mainly include oxides such as Al₂O₃, MgO, and SiO₂, as well as carbides and nitrides, with typical sizes ranging from 5 to 100 μm. The presence of inclusions disrupts the continuity of aluminum alloys, leading to defects such as cracks and inclusion-related flaws in cast products. This significantly reduces material strength, ductility, and corrosion resistance, and has a particularly detrimental effect on subsequent forming processes such as rolling and extrusion.
3.2.1 Principles of Inclusion Removal
The core principle of inclusion removal is adsorption–flotation. Through the adsorption effect of refining agents or filtration media, fine inclusions in the melt are captured and agglomerated into larger particles, which can then be removed either by floating to the melt surface or by being intercepted by filtration media. According to the removal mechanism, inclusion removal technologies can be divided into two main categories: refining-agent-based removal and filtration-based removal.
3.2.2 Mainstream Inclusion Removal Processes
Refining-Agent-Based Inclusion Removal
In this process, solid or gaseous refining agents are added to molten aluminum, and inclusions are removed through physical and chemical interactions between the refining agent and the inclusions. Refining agents are commonly classified into chloride-based (such as NaCl–KCl), fluoride-based (such as Na₃AlF₆), and composite refining agents (such as NaCl–KCl–Na₃AlF₆).
Their mechanisms of action include:
Adsorption effect: After melting, the refining agent forms a slag phase with a large specific surface area, which can adsorb fine inclusions present in the molten aluminum.
Chemical reaction: Certain refining agents can chemically react with inclusions to form compounds that are easier to float and separate. For example, Na₃AlF₆ can react with Al₂O₃ to form NaAlO₂, facilitating inclusion removal.
Refining-agent-based inclusion removal features simple operation and low cost and is suitable for the production of various aluminum alloys. However, its effectiveness is limited, and it is difficult to remove very fine inclusions (≤10 μm).
Filtration-Based Inclusion Removal
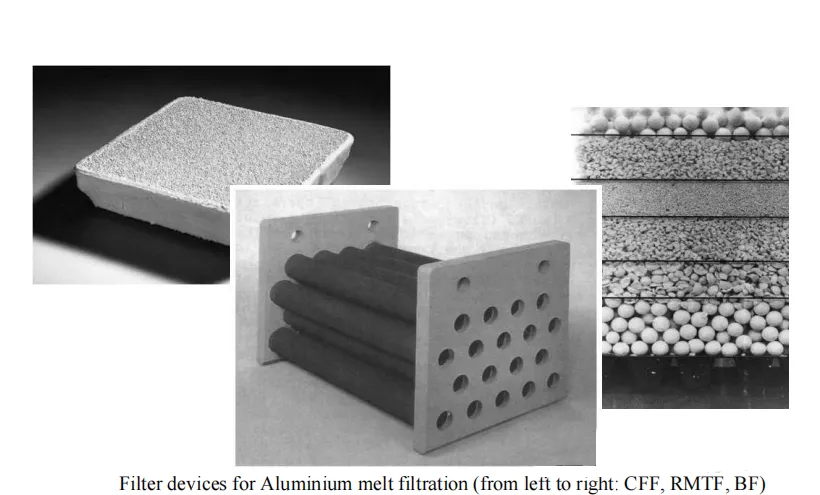
Filtration utilizes the interception effect of filtration media to separate inclusions from molten aluminum and is currently the most effective technology for deep inclusion removal. Common filtration media include ceramic foam filters, ceramic tube filters, and fiber filters, among which ceramic foam filters are the most widely used.
The working principle is as follows: when molten aluminum flows through the porous structure of a ceramic foam filter, inclusions are intercepted by the filter pores, while clean molten aluminum passes through, thereby achieving melt purification.
Ceramic foam filters typically have pore densities of 30–60 ppi (pores per inch). Smaller pore sizes provide higher filtration accuracy but also result in higher flow resistance. Therefore, appropriate pore size selection must consider melt flow rate and inclusion content. This process can effectively remove inclusions with sizes ≥10 μm, achieving inclusion removal efficiencies of over 90%, and significantly improving melt cleanliness. It is widely used in the production of high-end aluminum alloy castings and wrought products.
3.2.3 Key Control Parameters
Refining agent selection: Appropriate refining agents must be selected according to alloy type. For example, aluminum alloys containing magnesium should avoid chlorine-containing refining agents to prevent reactions that form volatile MgCl₂ and lead to magnesium loss.
Refining agent addition amount: Insufficient addition results in poor inclusion removal, while excessive addition increases slag volume and contaminates the melt. Typically, the addition amount is controlled at 0.1%–0.5% of the melt mass.
Filtration temperature: If the filtration temperature is too low, melt fluidity decreases and filter clogging may occur; if too high, melt oxidation risk increases. Filtration temperature is usually controlled within 700–750 °C.
Filtration speed: Excessively high filtration speed prevents effective interception of inclusions, while excessively low speed reduces production efficiency. Filtration speed is typically controlled within 0.5–1.5 m/min.
3.3 Composition Adjustment: Precise Control of Alloy Properties
Composition adjustment refers to the process of adding alloying elements or modifying element contents in molten aluminum according to product requirements, so that the melt composition meets specified standards. The types and contents of alloying elements directly determine the mechanical properties, corrosion resistance, and processing performance of aluminum alloys. For example, A356.2 alloy requires a silicon content of 6.5%–7.5%, a magnesium content of 0.25%–0.45%, and a strontium content of 0.015%–0.035%. Precise control of these element contents is the key to ensuring product quality.
3.3.1 Composition Adjustment Methods
Master Alloy Addition Method
Intermediate alloy ingots with specified compositions (such as Al–Si, Al–Mg, and Al–Sr master alloys) are added into the molten aluminum. Through melting and stirring, the alloying elements are dissolved and uniformly distributed. This method features simple operation and accurate composition control, making it suitable for large-scale industrial production. However, the cost of master alloy ingots is relatively high.
Wire Feeding Method
Alloy wires (such as aluminum–strontium wire and aluminum–titanium–boron wire) are continuously fed into the melt using a wire feeder. The wire melts rapidly in the molten aluminum, enabling precise composition adjustment. This method offers high addition accuracy and sufficient reaction, effectively reducing alloy element burn-off and segregation. It is especially suitable for adding elements that are prone to oxidation and volatilization, such as strontium and magnesium, and has been widely applied in high-end aluminum alloy production.
Powder Addition Method
Alloy powders (such as titanium powder and boron powder) are injected into the melt using inert gas as a carrier to achieve composition adjustment. This method is suitable for adding small amounts of alloying elements. However, alloy powders are prone to oxidation, and therefore strict control of the operating environment is required.
3.3.2 Key Control Parameters
Alloy element burn-off rate: Certain alloying elements, such as strontium, magnesium, and zinc, are susceptible to oxidation and burn-off at high temperatures. Therefore, addition amounts must be adjusted according to burn-off rates. For example, the burn-off rate of strontium is typically 30%–50%, requiring excess addition to ensure that the final content meets specifications.
Mixing uniformity: After alloying elements are added, mechanical stirring or gas stirring is required to ensure uniform composition distribution and prevent segregation. The typical stirring time ranges from 5 to 10 minutes.
Composition inspection: Real-time composition analysis of the melt is performed using spectrometric instruments such as direct-reading optical emission spectrometers. Based on the analysis results, alloying element additions are adjusted to ensure that the melt composition complies with standard requirements.
3.4 Grain Refinement: Optimization of Solidification Structure
Grain refinement refers to the process of adding grain refiners to molten aluminum in order to refine the solidification microstructure through heterogeneous nucleation. The grain size of aluminum alloys has a direct impact on their mechanical properties. A fine-grained structure can significantly improve the strength, toughness, and ductility of materials, while also enhancing crack resistance and processing performance of castings. In untreated aluminum alloys, grain sizes are typically on the order of several hundred micrometers, whereas after grain refinement, grain sizes can be reduced to below 50 μm.
3.4.1 Principles of Grain Refinement
The core principle of grain refinement is heterogeneous nucleation. Fine particles generated by the decomposition of grain refiners in the molten aluminum, such as TiAl₃ and TiB₂, can act as nucleation sites during solidification. These particles promote the formation of a large number of fine grains instead of a small number of coarse grains. Because the lattice parameters of these nucleation particles are close to those of aluminum, the nucleation energy barrier is reduced, thereby facilitating nucleation and achieving grain refinement.
3.4.2 Mainstream Grain Refiners and Processes
Aluminum–Titanium–Boron (Al–Ti–B) Grain Refiners
Al–Ti–B grain refiners are currently the most widely used grain refinement agents. Typical compositions include Ti 5%–B 1% or Ti 3%–B 0.15%. The grain refinement mechanism involves the decomposition of the Al–Ti–B refiner in the melt to produce TiAl₃ and TiB₂ particles. TiAl₃ acts as an effective nucleation core, while TiB₂ promotes the uniform dispersion of TiAl₃ particles in the melt, further enhancing the grain refinement effect. This type of grain refiner is suitable for most aluminum alloys and exhibits significant refinement performance, reducing grain sizes to 30–50 μm.
Aluminum–Titanium–Carbon (Al–Ti–C) Grain Refiners
Al–Ti–C grain refiners are suitable for aluminum alloys containing elements such as zirconium and chromium. These elements tend to react with boron to form composite compounds such as TiB₂–ZrB₂, which reduce the effectiveness of Al–Ti–B refiners. The refinement mechanism of Al–Ti–C is similar to that of Al–Ti–B, but the addition of carbon improves the stability of the refiner and extends its effective refinement duration.
Grain Refiner Addition Processes
Grain refiners are typically added using wire feeding or master alloy addition methods. The addition amount is generally 0.1%–0.3% of the melt mass. After addition, sufficient stirring is required to ensure uniform distribution of the refiner in the melt. The grain refinement holding time is typically 5–10 minutes.
3.4.3 Key Control Parameters
Grain refiner selection: Appropriate grain refiners must be selected based on alloy composition. For aluminum alloys containing zirconium or chromium, Al–Ti–C refiners are preferred.
Addition amount control: Insufficient addition results in poor grain refinement, while excessive addition may lead to residual refiner particles acting as inclusions, thereby degrading material properties.
Treatment temperature: The decomposition and nucleation processes of grain refiners are highly temperature-sensitive. The treatment temperature is typically controlled within 720–750 °C. Excessively high temperatures can cause refiner fading, while excessively low temperatures hinder refiner dissolution and dispersion.
3.5 Melt Holding: Stabilization and Quality Assurance of the Melt
Melt holding refers to maintaining molten aluminum within a specified temperature range for a certain period of time after completing degassing, inclusion removal, composition adjustment, and grain refinement. The purpose of melt holding is to stabilize melt temperature and composition, allow refining reactions to fully complete, and ensure that the melt is in an optimal and stable state before casting. Proper melt holding is an essential step to guarantee consistency and reliability of aluminum alloy quality.
During the holding process, the melt undergoes physical and chemical stabilization. Gas content, inclusion distribution, and alloy element uniformity gradually reach equilibrium. If holding time is insufficient, incomplete reactions may result in unstable melt quality; if holding time is excessive, oxidation, hydrogen reabsorption, and grain refiner fading may occur, negatively affecting final product performance.
3.5.1 Functions of Melt Holding
The primary function of melt holding is temperature stabilization. After multiple treatment processes, melt temperature often fluctuates. Through holding, temperature gradients within the melt are reduced, ensuring uniform temperature distribution, which is critical for stable mold filling and solidification behavior during casting.
Another important function is compositional homogenization. During alloy element addition and grain refiner treatment, local concentration differences may exist. Holding allows sufficient diffusion of alloying elements, eliminating segregation and ensuring that the overall melt composition meets specification requirements.
In addition, melt holding promotes the flotation and separation of residual inclusions and refining slag. Under static conditions, inclusions with lower density than molten aluminum gradually float to the melt surface, further improving melt cleanliness.
3.5.2 Key Control Parameters
Holding temperature: Holding temperature is generally slightly lower than treatment temperature and is typically controlled within 700–730 °C. If the temperature is too low, melt fluidity deteriorates and casting defects may occur; if too high, oxidation and hydrogen pickup are intensified.
Holding time: Holding time is usually controlled between 10 and 30 minutes, depending on furnace capacity, alloy type, and cleanliness requirements. Excessive holding time increases energy consumption and quality risks.
Atmosphere control: Protective atmospheres such as argon or nitrogen are often applied during holding to reduce oxidation and hydrogen absorption from the surrounding environment. For high-quality aluminum alloy production, flux covering is also commonly used to isolate the melt from air.
Melt surface management: Oxide films and slag on the melt surface must be removed regularly during holding to prevent them from being entrained into the melt during pouring.
4. Core Equipment for Aluminum Alloy Melt Treatment
The effectiveness of aluminum alloy melt treatment is closely related to the performance of the equipment used. Different treatment processes correspond to specialized core equipment. The following introduces the most commonly used key equipment in industrial production.
4.1 Degassing Equipment
Rotary Degasser:
The rotary degasser mainly consists of a graphite rotor, a rotation drive system, a gas supply system, and a control system. The graphite rotor is made of high-purity graphite, resistant to high temperatures and corrosion by molten aluminum. The rotation drive system allows precise speed control (0–1000 r/min). The gas supply system is equipped with a flow controller to ensure stable gas flow. The control system can automate the regulation of rotor speed, gas flow, and treatment time. Some high-end systems can also integrate with composition detection equipment to achieve closed-loop control.
Vacuum Degasser:
The vacuum degasser consists primarily of a vacuum furnace body, a vacuum pumping system, a heating system, and a control system. The furnace body is sealed and capable of withstanding high vacuum levels (typically 10–100 Pa). The vacuum pumping system quickly achieves the required vacuum. The heating system maintains melt temperature to prevent excessive temperature drop during holding. The control system automates the regulation of vacuum level, temperature, and processing time.
4.2 Inclusion Removal Equipment
Ceramic Foam Filter (CFF):
Composed of ceramic foam filter plates, a filtration box, and a temperature control system. The ceramic foam filter plates are made from Al₂O₃–SiO₂–ZrO₂ composite materials, offering high-temperature resistance and corrosion resistance, with pore sizes ranging from 10–50 ppi. The filtration box is steel-structured and lined with insulating materials to maintain melt temperature stability during filtration. The temperature control system maintains the temperature of the filtration box via heating devices, preventing rapid cooling that could reduce melt fluidity.
Refining Agent Injector:
This device consists of a spray gun, refining agent supply system, gas supply system, and control system. The spray gun is made of high-temperature-resistant alloy and can deliver refining agents deep into the melt. The supply system accurately controls the addition of refining agents. The gas system provides injection force to ensure uniform dispersion. The control system automates regulation of addition amounts and injection speed.
4.3 Composition Adjustment and Grain Refinement Equipment
Wire Feeder:
Consists of a wire feeding mechanism, drive system, guiding mechanism, and control system. The feeding mechanism uses a roller design to ensure stable wire delivery. The drive system employs a servo motor with precise speed control (0.1–5 m/min). The guiding mechanism ensures accurate delivery of wire into the melt. The control system automates wire feeding speed and length, and some systems can integrate with spectrometers to adjust feeding based on composition analysis in real time.
Master Alloy Charger:
Composed of a feeding mechanism, weighing system, and control system. The feeding mechanism uses robotic arms or conveyors to automatically add master alloy ingots. The weighing system ensures precise control of the addition, with an error margin ≤ ±1%. The control system calculates and adds the required amount of master alloy based on melt mass and composition requirements.
4.4 Holding Furnaces
Resistance Holding Furnace:
Composed of a furnace body, resistance heating elements, insulation layer, and control system. The furnace body is steel-structured and lined with refractory materials. The resistance heating elements are made of Ni–Cr alloys, offering high heating efficiency and long service life. The insulation layer uses ceramic fiber for excellent heat retention, reducing energy consumption. The control system allows precise temperature control (error ≤ ±5 °C).
Gas Holding Furnace:
Composed of a furnace body, burners, insulation layer, and control system. The burners utilize high-efficiency combustion technology for complete burning with low energy consumption. The insulation layer is similar to that of the resistance holding furnace. The control system automates regulation of temperature and combustion efficiency, making it suitable for large-scale production.
5. Technical Challenges and Innovation Directions in Recycled Aluminum Melt Treatment
5.1 Technical Challenges
Recycled aluminum, an important component of the circular economy, has advantages including resource conservation, low energy consumption, and reduced carbon emissions. However, recycled aluminum feedstock has complex composition and high impurity content (e.g., fluctuating levels of Fe, Si, Mn), which presents significant challenges for melt treatment:
Impact of Impurity Elements: Recycled aluminum typically contains high levels of Fe, which forms brittle Al₃Fe phases with Al, reducing material toughness and ductility. Fluctuating Si content affects alloy fluidity and mechanical performance, necessitating melt treatment to neutralize or remove impurities.
High Gas and Inclusion Levels: Recycled aluminum often has surface contaminants such as oil and oxide films. During melting, large amounts of hydrogen and non-metallic inclusions are generated, making degassing and inclusion removal more difficult than with primary aluminum.
Composition Variability: Recycled aluminum feedstock comes from diverse sources with large compositional differences. This causes frequent fluctuations in melt composition, requiring constant adjustment of alloying additions and increasing control complexity.
5.2 Innovation Directions
To overcome the technical bottlenecks in recycled aluminum melt treatment and promote high-end applications, the industry has developed a series of innovations:
Multi-Impurity Cooperative Removal Technology: Development of high-efficiency composite fluxes capable of simultaneously removing metallic impurities (Fe, Si) and non-metallic inclusions. For example, adding Ca or Mg to form low-melting-point compounds with Fe, which are then removed by flotation.
Intelligent Melt Treatment Systems: Integration of online composition, gas, and inclusion monitoring devices with automated processing equipment, enabling a closed-loop “detection–adjustment–treatment” system for precise melt quality control.
Green and Energy-Saving Melt Treatment: Development of low-temperature melt treatment processes to reduce energy consumption and alloy burn-off. Use of waste heat recovery systems to improve energy efficiency, and eco-friendly refining agents to reduce pollutant emissions.
100% Recycled Aluminum Precision Control Technology: For fully recycled aluminum with large compositional fluctuations, adaptive composition adjustment systems are being developed. By analyzing large datasets, a correlation model of “raw material composition–treatment parameters–melt quality” is established, achieving stable control of melt quality.
6. Conclusions and Outlook
Aluminum alloy melt treatment is a core process for improving quality and efficiency in the aluminum processing industry. The level of melt treatment technology directly determines product quality and market competitiveness. This paper systematically discusses the principles, key process steps (degassing, inclusion removal, composition adjustment, grain refinement, melt holding), core equipment, and industrial applications of aluminum alloy melt treatment, and analyzes technical challenges and innovation directions for recycled aluminum.
With increasing quality requirements in high-end manufacturing and the ongoing implementation of the “dual carbon” policy, aluminum alloy melt treatment technology is moving toward intelligent, green, and precise development. Future efforts should focus on:
Developing efficient, low-cost multi-impurity cooperative removal technologies to overcome the bottleneck in high-end recycled aluminum.
Building intelligent melt treatment systems to achieve fully automated “detection–adjustment–treatment” control.
Advancing green and energy-saving technologies to reduce energy consumption and carbon emissions during melt treatment.
Strengthening fundamental research to deepen understanding of gas and inclusion behavior in the melt, providing theoretical support for technological innovation.
Continuous innovation and application of aluminum alloy melt treatment technology will drive the industry toward high-quality, green, and high-end development, supporting upgrades in automotive, aerospace, and new energy sectors, and contributing to the achievement of the dual carbon goals and the growth of the circular economy.
Post time: Jan-24-2026